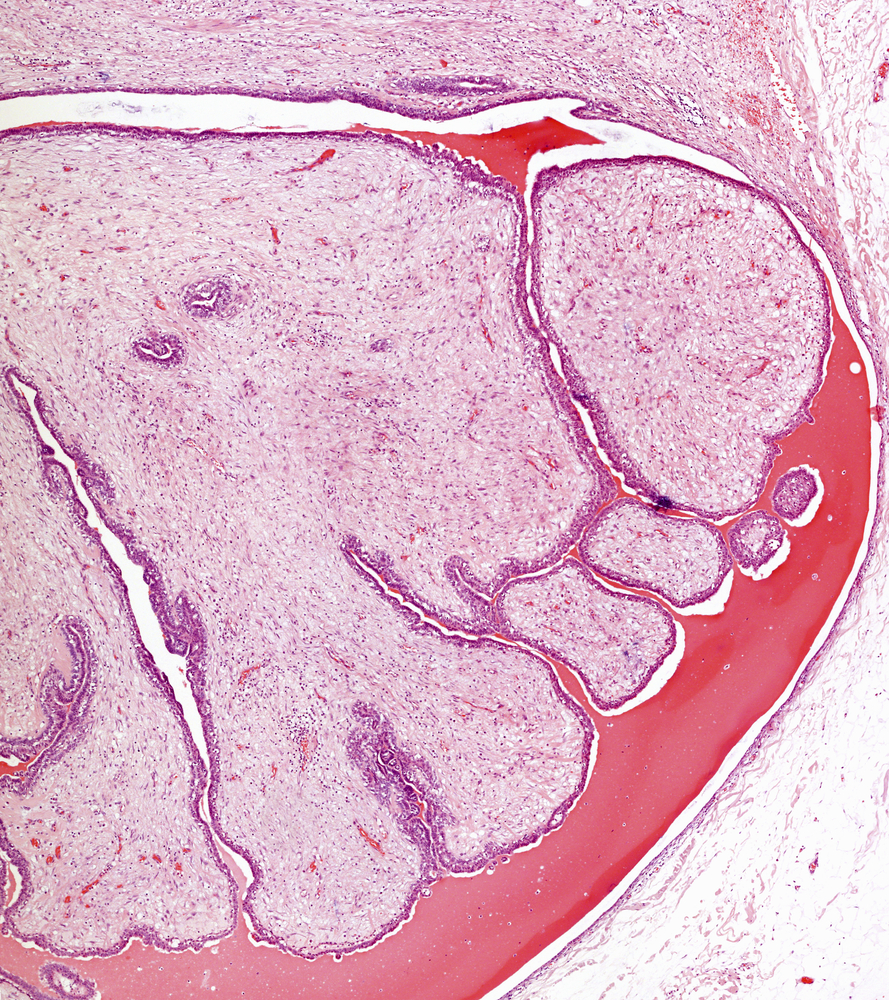
 Dr. Martha R. Stampfer’s laboratory at Lawrence Berkeley National Laboratory took a new approach to generating immortalized human mammary epithelial cells and now has “a very good model of a critical step in cancer progression,” according to her colleague, Dr. James C. Garbe. The two were on a team at Berkeley trying to develop a more useful model of early cancer progression that could be used as a platform to explore therapeutics directed against tumor development.
Dr. Martha R. Stampfer’s laboratory at Lawrence Berkeley National Laboratory took a new approach to generating immortalized human mammary epithelial cells and now has “a very good model of a critical step in cancer progression,” according to her colleague, Dr. James C. Garbe. The two were on a team at Berkeley trying to develop a more useful model of early cancer progression that could be used as a platform to explore therapeutics directed against tumor development.
“We believe that research on these cells may stimulate new approaches for therapeutic intervention in cancer progression at the earliest stages,” said Dr. Garbe in a news release from Berkeley. The team’s findings regarding this approach were published as “Immortalization of Normal Human Mammary Epithelial Cells in Two Steps by Direct Targeting of Senescence Barriers Does Not Require Gross Genomic Alterations” in the journal Cell Cycle.
Most models of immortalized human cells rely upon genetic alterations to induce immortality. The altered genome can confound underlying reasons for cancer progression and mask errors responsible for immortalization due to overshadowing by “passenger” errors secondary to cancer development. Using mouse cells does little more to improve validity, as normal mouse cells already express enzymes that are part of immortality.
The group at Berkeley decided to take a different route by focusing on cellular senescence. By halting a pathway that stops cell division, the team thought they could grant immortality to cells without touching the genome. “That’s important. It allows us to study the mechanisms underlying immortalization as it occurs in cells with normal genomes,” said Dr. Garbe. “We believe that research on these cells may stimulate new approaches for therapeutic intervention in cancer progression at the earliest stages.”
[adrotate group=”3″]
To begin, the team knocked down p16, a protein that puts the breaks on cell division, using short hairpin RNA (shRNA). After, they induced c-Myc activity, increasing telomerase activation. This step allowed cells to maintain the protective caps on the ends of chromosomes to prevent DNA damage-induced cellular senescence. These two steps were an easy, reproducible means of generating immortal cells.
“Now that we have these immortal human mammary epithelial cells with normal genomes, we can study them to explore the molecular mechanisms behind cell immortality, from start to finish,” said Dr. Stampfer. “We can also begin to think about ways to target this process therapeutically in order to prevent or reverse cancer progression.”