 Findings from a recent study published in the Journal of Clinical Oncology, indicate that FDA approved Herceptin (trastuzumab) can improve long-term survival of HER-2 positive breast cancer patients.
Findings from a recent study published in the Journal of Clinical Oncology, indicate that FDA approved Herceptin (trastuzumab) can improve long-term survival of HER-2 positive breast cancer patients.
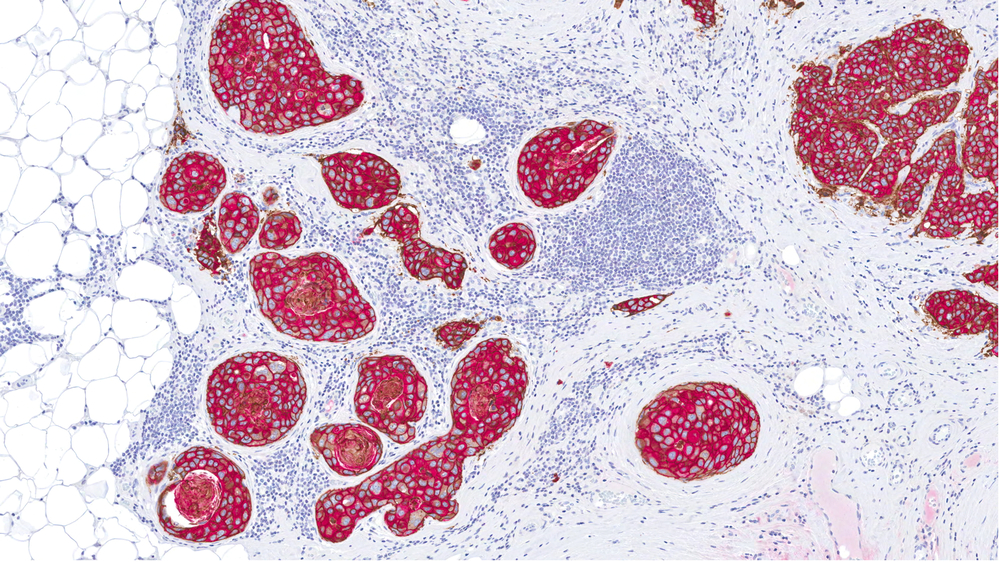
Approximately 15% to 20% of invasive breast cancers have an amplification of the human epidermal growth factor receptor 2 (HER2) gene or overexpression of the HER2 protein.
Trastuzumab, a monoclonal antibody that can target the extracellular domain of the HER2 protein, has been found to improve survival in the metastatic setting of the disease when used in combination with chemotherapy.
The NCCTG N9831 (hereafter N9831) and NSABP B-31 (hereafter B-31) trials assessed the efficacy and safety of adding trastuzumab to paclitaxel followed by trastuzumab alone after completion of doxorubicin and cyclophosphamide chemotherapy (doxorubicin and cyclophosphamide, trastuzumab plus paclitaxel, trastuzumab).
The trials were similar in design, allowing the National Cancer Institute and the US Food and Drug Administration to approve a joint efficacy analysis plan that could be executed before the first planned efficacy interim analysis of the individual trials.
In their study entitled “Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831”, a team of researchers from the VCU Massey Cancer Center, analyzed a total of 4,046 patients with HER2-positive operable breast cancer who received doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab.
The team found that combining trastuzumab to paclitaxel after doxorubicin and cyclophosphamide in early-stage HER2-positive breast cancer, improved 10-year survival from 75% with chemotherapy alone to 84% when trastuzumab was added. Moreover, the results showed a sustained marked reduction in cancer recurrence.
[adrotate group=”3″]
Toxic side effects that usually occur with Herceptin, such as heart problems, were only present in 3% of the patients and were rapidly resolved.
In a recent news release, Dr. Charles E. Geyer, senior scientific advisor to the NSABP and professor in the Division of Hematology, Oncology and Palliative Care at the VCU School of Medicine, said “We have found that when Herceptin is used in combination with chemotherapy, a patient’s survival is significantly improved. There are minimal long-term side effects, and the likelihood of the cancer recurring is greatly reduced.”
Currently other clinical trials are examining patients’ outcomes using herceptin combined with other compounds that target HER-2 positive breast cancer.