Devicor Medical Products, a division of Leica Biosystems, is now the exclusive United States and Canada distributor of Endomag’s magnetic surgical guidance system, Sentimag. The technology used in Sentimag is heralded as raising the bar for standard of care of breast cancer patients undergoing surgical procedures.
Devicor Medical Products, a division of Leica Biosystems, is now the exclusive United States and Canada distributor of Endomag’s magnetic surgical guidance system, Sentimag. The technology used in Sentimag is heralded as raising the bar for standard of care of breast cancer patients undergoing surgical procedures.
 Founded in 2007 as a spin-off from the University College London (UCL) and the University of Houston, Cambridge, Endomag sells its products throughout Europe, the Middle East, Africa and Australasia, and is seeking authorization to expand globally.
Founded in 2007 as a spin-off from the University College London (UCL) and the University of Houston, Cambridge, Endomag sells its products throughout Europe, the Middle East, Africa and Australasia, and is seeking authorization to expand globally.
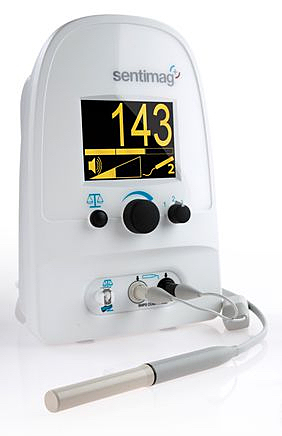 Sentimag, a handheld magnetic probe and the core element of the system, is engineered to detect minuscule amounts of magnetic material in the body, in order to guide surgeons performing lumpectomies using the company’s Magseed magnetic tracer product. The product can be placed by radiologists up to 30 days prior to the surgery allowing greater scheduling flexibility for surgeons and radiologists with wire-guided localization.
Sentimag, a handheld magnetic probe and the core element of the system, is engineered to detect minuscule amounts of magnetic material in the body, in order to guide surgeons performing lumpectomies using the company’s Magseed magnetic tracer product. The product can be placed by radiologists up to 30 days prior to the surgery allowing greater scheduling flexibility for surgeons and radiologists with wire-guided localization.
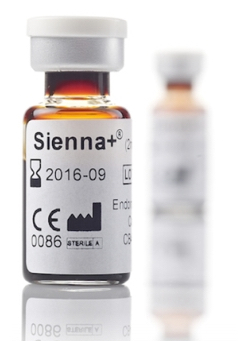 Sentimag can also be used to scan the lymph nodes first in line by using Sienna+, a proprietary, non-toxic, suspension solution of superparamagnetic iron oxide particles injected where the lymphatic system’s natural physical action filters out the particles. The sentinel nodes can then be detected in tiny quantities using the Sentimag. This system replaces radioactive tracers and blue dyes used in sentinel lymph node biopsies. Localization can start after only 20 minutes and last up to seven days following injection.
Sentimag can also be used to scan the lymph nodes first in line by using Sienna+, a proprietary, non-toxic, suspension solution of superparamagnetic iron oxide particles injected where the lymphatic system’s natural physical action filters out the particles. The sentinel nodes can then be detected in tiny quantities using the Sentimag. This system replaces radioactive tracers and blue dyes used in sentinel lymph node biopsies. Localization can start after only 20 minutes and last up to seven days following injection.
Sentimag received European Community authorization in 2010, Sienna+ was authorized in 2011, and has been used in more than 14,000 procedures throughout Europe. Sentimag devices and Sienna tracer are also used in the Caribbean, Hong Kong, Singapore and Australasia — made up of Australia, New Zealand, the island of New Guinea, and neighboring Pacific Ocean islands.
Findings from a multi-center clinical trial of the Endomag products in the U.S., completed in 2015, formed the basis of a Pre-Market Approval (PMA) submission to the FDA earlier this year. The products received FDA 510(k) clearance in March; the company says CE mark approval is expected before the end of this summer.
The Devicor Medical Products distribution agreement will expand US and Canadian access to care.
 “We are very excited to be the exclusive distributor of the Sentimag system in the United States and Canada,” said Alain de Lambilly, president of Leica Biosystems Tissue Acquisition Solutions in a press release. “This technology represents a major advancement in breast cancer procedures for clinicians and helps to improve the overall standard of care received by patients everywhere.”
“We are very excited to be the exclusive distributor of the Sentimag system in the United States and Canada,” said Alain de Lambilly, president of Leica Biosystems Tissue Acquisition Solutions in a press release. “This technology represents a major advancement in breast cancer procedures for clinicians and helps to improve the overall standard of care received by patients everywhere.”
The Devicor Medical Products-Endomag agreement includes the Sentimag system, Magseed, and Sienna in both the United States and Canada, pending regulatory approval within those markets.
 “Leica Biosystems is a leader in breast care, with products like the Mammotome breast biopsy system,” said Eric Mayes, PhD, CEO of Endomag. “We are truly thrilled for them to offer our products alongside theirs in the U.S. and Canada. They have a world-class team and genuinely share our passion for improving lives, so are a natural partner to improve the standard of cancer care for everyone, everywhere.”
“Leica Biosystems is a leader in breast care, with products like the Mammotome breast biopsy system,” said Eric Mayes, PhD, CEO of Endomag. “We are truly thrilled for them to offer our products alongside theirs in the U.S. and Canada. They have a world-class team and genuinely share our passion for improving lives, so are a natural partner to improve the standard of cancer care for everyone, everywhere.”