 During the 2014 Plastic Surgery annual meeting of the American Society of Plastic Surgeons (ASPS), AirXpanders Inc.’s will present its latest data from XPAND, a head-to-head pivotal study of AeroForm, the company’s needle-free tissue expansion system for mastectomy patients undergoing breast reconstruction.
During the 2014 Plastic Surgery annual meeting of the American Society of Plastic Surgeons (ASPS), AirXpanders Inc.’s will present its latest data from XPAND, a head-to-head pivotal study of AeroForm, the company’s needle-free tissue expansion system for mastectomy patients undergoing breast reconstruction.
The conference, currently taking place in Chicago, will also be the stage for the presentation of several separate analyses and studies investigating the impact of air travel and radiation therapy on patients with the AeroForm expanders implanted.
Women diagnosed with breast cancer who have to undergo mastectomy and want to have breast cancer reconstructive surgery can face a lengthy and painful process with the commonly used, needle-based, saline tissue expanders.
To address this unmet medical need, AirXpanders developed the AeroForm tissue expander, a patient controlled breast tissue expander that comes equipped with a small handheld wireless controller that administers small amounts of CO2 into the device, stretching the skin to accommodate a permanent breast implant. This method eliminates the need for percutaneous saline injections, shortens tissue expansion time from months to weeks, reduces pain and discomfort experienced by patients, can improve cosmetic outcomes for a natural form and shape and reduces overall time and costs.
[adrotate group=”3″]
“Breast reconstruction following a mastectomy is long process, and tissue expansion is traditionally is one of the most tedious and painful aspects. For women to achieve expansion in 18.7 days on average and for the vast majority of the patients to report high satisfaction throughout the expansion process is extremely compelling, and further supports AeroForm as an exciting, much-needed innovation in breast reconstruction”, Kamakshi Zeidler, M.D., principal investigator of the XPAND trial said in a company’s press release. “Patient-controlled expansion, where women are able to expand at their own pace and level of comfort, offers mastectomy patients a needle-free, more convenient option for expansion – and a chance to regain control over their body during a time in which much is out of their control. It is my expectation that with 98% of the women in the early phase of the study reporting that the AeroForm is easy to use, CO2-based expanders have the potential to be adopted universally, and transform the process of tissue expans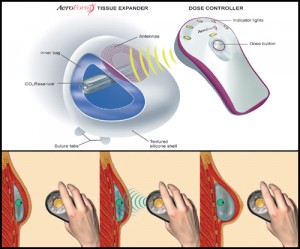 ion.”
ion.”
Researchers have reported that so far, 87 patients in the XPAND trial have successfully completed their expansion within an average of 18.7 days, which is a significant improvement over the 56.8 days the control group undergoing traditional saline injections took to complete expansion.
Congress speakers include Dr. Anthony Connell, The Mount Hospital, Perth Australia, presenting “The Future of Tissue Expansion For Two Stage Breast Reconstruction Procedures”; Dr. Jeffrey Ascherman, Columbia Presbyterian Medical Center, presenting “XPAND Patient Activated Controlled Tissue Expansion System: A U.S, Multi-centered, Randomized Controlled Trial”; and Dr. Anthony Connell, The Mount Hospital, Perth Australia, presenting “The Use of Radiotherapy and Altitude Effects on the AeroForm Tissue Expander”.

