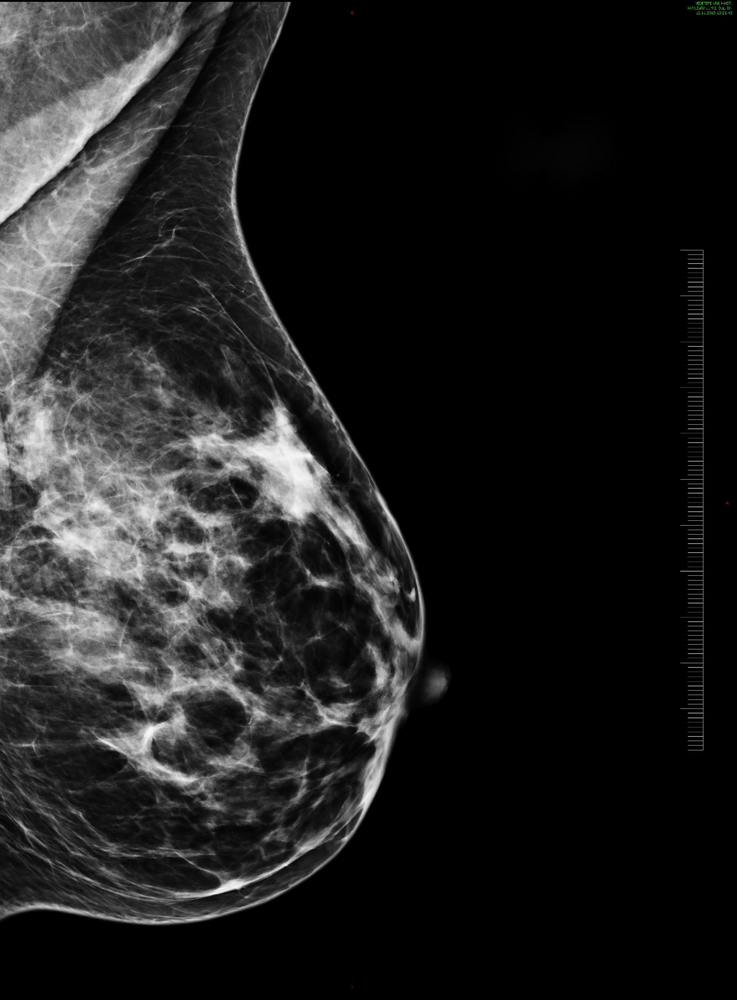
 Navidea Biopharmaceuticals, Inc. will now be able to market its product, Lymphoseek (technetium Tc 99m tilmanocept), as an injection for lymphatic mapping in solid tumors and sentinel lymph node (SLN) detection in breast cancer and melanoma. The Food and Drug Administration expanded its approval for these two cancers, increasing cancer types beyond the previous head and neck cancers with squamous cell carcinoma of the oral cavity, and also approved Lymphoseek with or without scintigraphic imaging to map lymph nodes pre-operatively to facilitate locating nodes during surgery.
Navidea Biopharmaceuticals, Inc. will now be able to market its product, Lymphoseek (technetium Tc 99m tilmanocept), as an injection for lymphatic mapping in solid tumors and sentinel lymph node (SLN) detection in breast cancer and melanoma. The Food and Drug Administration expanded its approval for these two cancers, increasing cancer types beyond the previous head and neck cancers with squamous cell carcinoma of the oral cavity, and also approved Lymphoseek with or without scintigraphic imaging to map lymph nodes pre-operatively to facilitate locating nodes during surgery.
“We are highly encouraged by the expanded FDA approval and believe that Lymphoseek now has the potential to become a standard-of-care in lymphatic mapping and SLN biopsy for the staging and prognosis of upwards of 1.2 million patients diagnosed with solid tumors annually in the US,” said Michael Goldberg, MD, interim chief executive officer of Navidea, in a company’s news release.
Approval was contingent upon several phase 3 clinical trials comparing Lymphoseek to vital blue dye (VBD), the current standard for node detection. Two studies of 148 total breast cancer patients indicated an agreement between the two detectors of 99.04%. Of the 209 SLNs detected by VBD, Lymphoseek detected 207. Another two studies with 154 total melanoma patients showed an agreement rate of 98.7%. Lymphoseek detected 232 of the 235 SLNs detected with VBD. However, Lymphoseek detected all 45 melanoma-positive SLNs, whereas VBD detected only 80%.
Injection site irritation was reported by only four (0.7%) patients, and only one (0.2% patient reported patin at the injection site. No serious adverse events have been reported with the use of Lymphoseek.
“The ability of Lymphoseek to accurately identify SLNs in patients, demonstrated in clinical evidence from more than 500 patients, may not only improve diagnostic accuracy, but also enable more efficient and approriate patient care and provide us with greater precision during surgery to detect lymph nodes with the highest likelihood of harboring tumor metastases,” said Stephen Y. Lai, MD, PhD, FACS, an associate professor at the University of Texas MD Anderson Cancer Center.
Lymphoseek is the only FDA-approved radiopharmaceutical agent that can be used to detect SLN and map lymphatics of solid tumors. After an injection of Lymphoseek, a hand-held gamma counter is used to localize lymph nodes that drain a primary tumor site by binding to receptors within lymph nodes. During studies, Lymphoseek could be detected in lymph nodes within 10 minutes and up to 30 hours after injection, but it is recommended lymphatic mapping be conducted between 15 minutes and 15 hours after injection.
[adrotate group=”3″]
Although SLN mapping with Lymphoseek has been approved for only a few cancers, H. William Strauss, MD, of Memorial Sloan Kettering Cancer Center, stated, “Based on the reliable performance of Lymphoseek as demonstrated in [breast cancer, melanoma, and certain head and neck cancers], this approval opens up potential diagnostic imaging opportunities broadly across all forms of solid tumors.”
One of the next items on the agenda for Navidea is to conduct a post-marketing study of pediatric patients with solid tumors. Submission for the trial to the FDA is expected by 2018.