The Center for Environmental Health (CEH) has filed a legal petition to the California Department of Toxic Substances Control (DTSC) demanding that the state Safer Consumer Products program regulate BPA (Bisfenol A) in canned food and beverages.
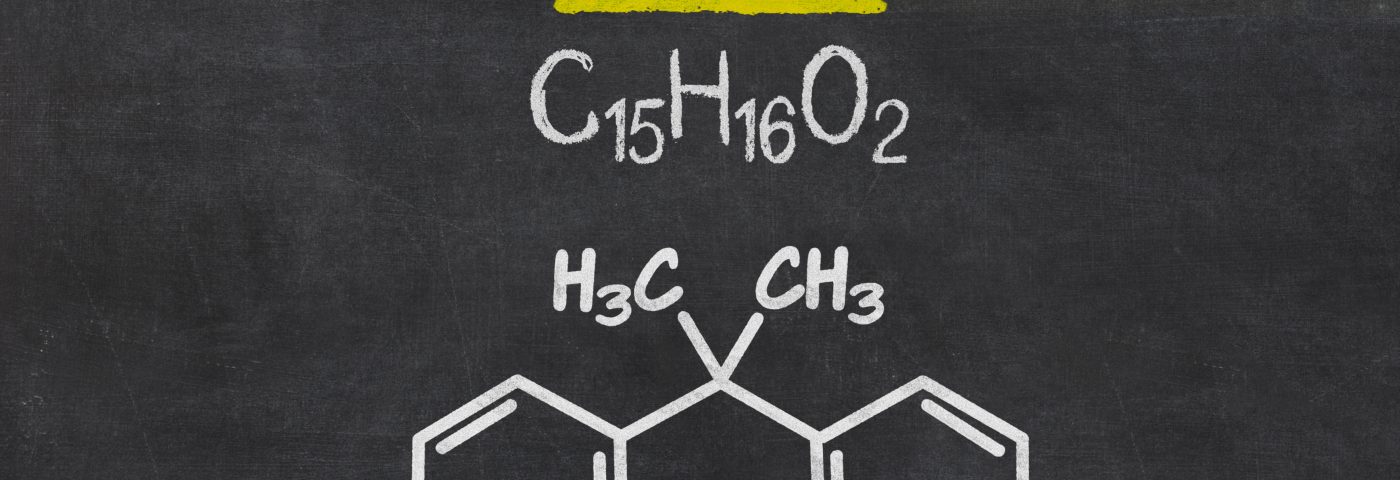
BPA is a controversial chemical produced in large quantities for use in the production of polycarbonate plastics and epoxy resins. Polycarbonate plastics are used in products like food and drink packaging – such as water and infant bottles, safety equipment, and medical devices. Epoxy resins are usually found in lacquers to coat metal products – like food cans, bottle tops, and some dental sealants and composites.
BPA has been linked to birth defects, breast cancer, prostate cancer, low sperm counts, abnormal sexual development in males, early sexual maturation in females, increasing prevalence of obesity, and damage to the immune system.
“The state has denied consumers the right to know when canned food contains this dangerous chemical, despite long-established rules that require companies to warn consumers about this toxic risk,” said Michael Green, CEH’s chief executive officer, in a press release. “We are calling on the state to end this assault on consumer choice. There is no excuse for denying us our right to know when harmful chemicals are in our food.”
The petition is grounded on the fact that last year California added BPA to its Proposition 65 list of chemicals known to cause birth defects, and then notified companies to begin warning consumers about their products that use the compound by until May 2016.
However, just weeks before the deadline, an ‘emergency’ rule was released, without previous public comment or notice, allowing canned food companies to continue using BPA in their products without warning consumers. In addition, last month the state proposed to extend the emergency rule, which would allow companies to continue to keep consumers in the dark at least through December 2017.
CEH is concerned that the extension could undermine almost three decades of legal precedence that has successfully protected Californian children and families from harmful chemicals.
CEH’s petition included a study from the Centers for Disease Control and Prevention showing that almost all Americans (93%) have BPA in their bodies. The U.S. Food and Drug Administration (U.S. FDA) also surveyed the consumption of canned food and found that it is the source of most exposure toBPA nationwide.
A public hearing on the proposed new BPA rule is scheduled for 10 a.m., September 12, in the Sierra Hearing Room at the CalEPA Headquarters, 1001 I Street, Sacramento, Calif. The hearing will also be webcasted here.
To take immediate action against BPA chemical impact, here is some advice from the National Institutes of Health:
- Don’t microwave polycarbonate plastic food containers. Polycarbonate is strong and durable but over time it can break down from over use at high temperatures;
- Plastic containers have recycle codes on the bottom but some are marked with recycle codes 3 or 7 – these might be made with BPA;
- Reduce your use of canned foods all together;
- Whenever possible, opt for glass, porcelain or stainless steel containers, especially for hot food or liquids;
- Look for baby bottles that specify being BPA free.
Click here to submit a written comment to the California Department of Toxic Substances Control (DTSC) through September 26.